The camp ran between December 6th and December 9th 2010 and involved one overnight camp / bivvy along the banks of the Waioeka River between Koranga Forks Hut and Wairata and two nights camping on Redpath's Farm at Wairata.
The overnight camp out was in the middle of a tramp from Moanui Valley along the Koranga and down the Waoieka to Wairata. Some groups, with prior planning and foresight, managed to get as far as Nikau Flats Hut which meant no more river crossings on the last day's tramp. The weather gods played ball with nice sunny weather and low levels for the numerous river crossings along the tramp. This, however, meant that the Waioeka was a little bony for the kayaking and rafting the next day. To use and abuse a quote from some of my paddling acquaintances: 'it was low and (not really) doable!', but we got down through Nutcracker and Corkscrew along with the rest of the river, albeit it with the occasional boat walk! The last morning dawned bright and sunny which was a great opportunity to dry out tents form some of the showers the day before, and even more importantly do the abseiling off the bridge in the sun! Below is a slide show of some of the photos I took on the camp:
Thanks, in no particular order, for such a successful camp must go to the following: the Redpath's for the use of the campsite, the GBHS year 10 Boys on the camp, the GBHS staff who ran/supervised the camp and activities, and the other staff and helpers..
School Banner

School Logo
Monday, December 13, 2010
Friday, November 19, 2010
PERIODIC TABLE
This is the start, middle and end of trying to understand chemistry and how and why elements react:
There are a lot of elements here and first impressions maybe that trying to remmber the elements in order up to the first 20 is too difficult....wrong!! This is where mnemonics come in!
Hopata, - He - Like - Beer, - Bottled - Cold, - Not - Over - Frothy, - Nelly -(the) - Naughty - Might - Although - Silly - Person, - She - Climbs - Around - Kinky - Caves.
-Standing for:
Hopata = Hydrogen
He = Helium
Like = Lithium
Beer = Beryllium
Bottled = Boron
Cold = Carbon
Not = Nitrogen
Over = Oxygen
Frothy = Fluorine
Nelly = Neon
the Naughty = Sodium
Might = Magnesium
Although = Aluminium
Silly = Silicon
Person = Phosphorous
She = Sulfur
Climbs = Chlorine
Around = Argon
Kinky = Potassium
Caves = Calcium
Using a mnemonic like this wil hopefully help with remebering the first 20 elements at least. You could even maybe com eup with one of your own!!
Here is another one for more elements...
- remember group numbers and valence shell electrons!!
There are a lot of elements here and first impressions maybe that trying to remmber the elements in order up to the first 20 is too difficult....wrong!! This is where mnemonics come in!
Hopata, - He - Like - Beer, - Bottled - Cold, - Not - Over - Frothy, - Nelly -(the) - Naughty - Might - Although - Silly - Person, - She - Climbs - Around - Kinky - Caves.
-Standing for:
Hopata = Hydrogen
He = Helium
Like = Lithium
Beer = Beryllium
Bottled = Boron
Cold = Carbon
Not = Nitrogen
Over = Oxygen
Frothy = Fluorine
Nelly = Neon
the Naughty = Sodium
Might = Magnesium
Although = Aluminium
Silly = Silicon
Person = Phosphorous
She = Sulfur
Climbs = Chlorine
Around = Argon
Kinky = Potassium
Caves = Calcium
Using a mnemonic like this wil hopefully help with remebering the first 20 elements at least. You could even maybe com eup with one of your own!!
Here is another one for more elements...
Labels:
9Si,
Chemical Reactions,
Chemistry,
Periodic Table,
Revision,
Valence Shells,
Year 10 Sia1,
Year 11 PY
Friday, October 22, 2010
11 PY CHEMISTRY REVISION..
Lewis Dot Diagrams continued...
For now just a quick post: CLICK HERE TO TRY WRITING LEWIS DOT DIAGRAMS FOR SOME COVALENT COMPOUNDS
For now just a quick post: CLICK HERE TO TRY WRITING LEWIS DOT DIAGRAMS FOR SOME COVALENT COMPOUNDS
YEAR 11PY CHEMISTRY REVISION
LEWIS DOT DIAGRAMS....
The basic starting point is understanding electron configuration of the elements and realising that we are only concerned with the outer shell of electrons as these electrons are the ones involved in bonding.
REMEMBER this is the valence shell and all elements in the same group have the same valency and the same Lewis Dot Diagram configuration!
1. Draw electron dot diagrams for elements for just the outer shell. The outer shell can contain 8 electrons-the OCTET RULE.
2. Electrons need to be drawn on the four sides of the Symbol on their own first
3. After you complete the four sides start to double up the electrons so they are in pairs. It doesn't matter where you start!
The next post / updated post will continue with Lewis Dot diagrams and focus on bonds between elements.....
In the meantime check out this site for an interactive slide show on Lewis Dot Diagrams of Covalent Bonds
The basic starting point is understanding electron configuration of the elements and realising that we are only concerned with the outer shell of electrons as these electrons are the ones involved in bonding.
REMEMBER this is the valence shell and all elements in the same group have the same valency and the same Lewis Dot Diagram configuration!
1. Draw electron dot diagrams for elements for just the outer shell. The outer shell can contain 8 electrons-the OCTET RULE.
2. Electrons need to be drawn on the four sides of the Symbol on their own first
3. After you complete the four sides start to double up the electrons so they are in pairs. It doesn't matter where you start!
 |
| Lewis Dot Diagrams for elements |
In the meantime check out this site for an interactive slide show on Lewis Dot Diagrams of Covalent Bonds
Wednesday, September 22, 2010
NCEA REPORT ON EXTERNAL ASSESSMENTS...11 PY
This is a shortened version of the NCEA Report on Exam results for the Standards assessed for 11 PY....some of the general points are of relevance to 10SiA1 as well-so read on Boys!!
General: Level 1 Chemistry
- Students who gained Achievement with Excellence wrote detailed answers demonstrating a good understanding of the concepts. In addition they had clearly carried out practical work and understood and remembered it as this was reflected in their answers
- Other successful students were clearly familiar with a wide range of experiments and were able to describe the observations made in these practicals using correct / appropriate terms, eg describing a solution as colourless not clear
- Writing Balanced Equations was required in many questions
- Remember that charges are required for the formulae of ions, eg Mg2+, but are not required for formulae of compounds, eg Mg(OH)2
- A common mistake was to write inferences and not actual observations, eg stating that CO2 gas forms as opposed to 'fizzing occured'. Carbon Dioxide gas may well have been given off but this was not observed, whereas fizzing or bubbling was observed: Write down observations!
- This is a common mistake:in many cases students did not give the required answers; indicating that they did not read the question carefully!
- Also remember to attempt all questions. Many students did not try all questions, eg only trying 2 questions in a 3 question paper, making it impossible to Achieve.
General: Level 1 Physics
- Most students can answer numerical questions reasonably well. However lack of full workings and understanding of Scientific terms etc prevented more students achieving Merit or Excellence
- Students who were able to express themselves clearly and accurately did well, with those who were able to respond appropriately to 'describe' and 'explain' answering the questions at the appropriate leve
- Some students did not read the questions properly which was reflected in their answers
Overall it is important to know how to do the following to make sure you have the best chance to do well in the NCEA Standards:
A/S 90171: Describe Chemical Reactions:
- Know Precipitation Rxns
- Know a range of practicals and the observations
- Write balanced equations, including ionic equations and the 'role' of spectator ions
- Know Redox Rxns, including the species being reduced and oxidised, know oxidation is the gain of oxygen and/or loss of electrons (reduction being the reverse) and discuss Redox rxns in terms of electron transfer
- Calculate Molar Masses
- Calculate empirical and molecular formulae
- Know the difference between ions and ionic compounds
A/S 90171: Describe Atomic Structure:
- Define Isotopes and the number of Neutrons
- Draw Lewis Diagrams
- Know the difference between ionic and covalent bonds and the importance of particle type and forces of attraction, and thus ionic and covalent compounds
- Know that mobile ions and charged particles are responsible for electrical conductivity
- Know that the electron configuration is linked to reactivity etc
A/S 90183: Demonstrate Understanding of Mechanics in 1 Dimension:
- Know the difference between vector and scalar quantities
- Know how to interpret velocity/time graphs, such as areas under the graph and gradient of the line...
- Identify forces acting on objects and the role of balanced and unbalanced forces and how these impact on motion
- Know how force, pressure, area interact
- Know that work done is dependent on distance covered and force applied
- Understand concepts of conservation of energy (Ep=Ek) and how to calculate distance travelled as well as calculating Ep
A/S 90185: Demonstrate Understanding of Electricity and Magnetism
- Know how objects are charged, only negative charges are displaced during charging in solid objects and the direction of motion of charges
- Know the differences between insulators and conductors
- Understand the relationship between Voltage, Current and Resistance (Ohms Law), how these affect Power Output (P=VI) and know the circuit patterns of voltage and current in series and parallel and how these affect eg brightness of lamps
- Understand Magnetism with respect to fields of attraction between poles, earth's magnetic field and how magnetism is related to electricity
- Know how to work out magnetic field strengths
10 top tips to help with the exams...(well hopefully!!)
- Pay attention in class to revision exercises / classes
- Organise study time at home and don't leave it all until the last minute!
- Practice past papers from the NCEA website and go over sample answer
- Aim for an Excellence before going for the exam and don't just try to scrape an Achieved
- Read each question properly at least once before answering, to make sure you understand what is being asked
- Use the correct units and show full workings with calculations
- Write more not less; you may be surprised and know more than you think you know
- Attempt every question and all parts of each question
- Go over your paper at the end when you think you have finished!
- Even check in with this Blog every so often
Labels:
Chemistry,
Credits,
Exams,
Externals,
NCEA,
Physics,
Revision,
Year 10 Sia1,
Year 11 PY
Thursday, September 9, 2010
MORE REVISION INFO FOR 10 a1 AND 11 SCIENCE
Remember 10 a1 (and 11 Science) the Standards needed for NCEA External Exams which will be in the Mock Senior Exams:
MICRO-ORGANISMS
Below is a 'simple' flash animation from the BBC Bitesize Science Website..
- Science 1.3 Describe Aspects of Biology
- Science 1.4 Describe Aspects of Chemistry
- Science 1.6 Describe Aspects of Physics
MICRO-ORGANISMS
- All micro-organisms are CONSUMERS, whereas Green Plants for example are producers, meaning they produce there own food. We are also consumers, like all animals, as we cannot produce our own food, instead we eat other organisms. Fungi do not contain chlorophyll so cannot produce their own food
- Micro-organisms are either SAPROPHYTES or PARASITES
- Saprophytes live and feed on dead matter and can be called decomposers
- Parasites live on living organisms
- Microorganisms are divided into: BACTERIA, FUNGI and VIRUS
Below is a 'simple' flash animation from the BBC Bitesize Science Website..
CLICK HERE FOR ALL YOU NEED TO KNOW FOR SCIENCE NCEA LEVEL 1
Here are some questions you may want to try:
1. When investigating whether microorganisms are present in air and soil
a) Why must the original petri dishes be sterile
b) Why is the lid left off one dish
c) Why the petri dishes are labelled on the bottom
d) Why are the dishes left in a warm place
e) How can you tell the difference between the different microorganisms growing on the plate
f) What types of microorganisms are growing on the plate and what are the reasons
g) Why was there no growth on the control plate
2. a) Name a way in which fungi are different to green plants
b) Why can fungi never act as producers in a food chain
c) Fungi are used to make bread. What conditions during bread making allow fungi to grow and help in he bread making process
3. a) Name 3 conditions needed for bacteria to grow
b) Give two benefits of bacteria
c) Name 3 diseases caused by bacteria
4. a) Discuss why viruses only exist as parasites
b) Why can viruses not be cultivated on an agar plate
c) How do viruses reproduce and what is this method called
ClICK HERE for Sample Answers....TRY AND GIVE THE Q'S A GO FIRST!!
Here are some questions you may want to try:
1. When investigating whether microorganisms are present in air and soil
a) Why must the original petri dishes be sterile
b) Why is the lid left off one dish
c) Why the petri dishes are labelled on the bottom
d) Why are the dishes left in a warm place
e) How can you tell the difference between the different microorganisms growing on the plate
f) What types of microorganisms are growing on the plate and what are the reasons
g) Why was there no growth on the control plate
2. a) Name a way in which fungi are different to green plants
b) Why can fungi never act as producers in a food chain
c) Fungi are used to make bread. What conditions during bread making allow fungi to grow and help in he bread making process
3. a) Name 3 conditions needed for bacteria to grow
b) Give two benefits of bacteria
c) Name 3 diseases caused by bacteria
4. a) Discuss why viruses only exist as parasites
b) Why can viruses not be cultivated on an agar plate
c) How do viruses reproduce and what is this method called
ClICK HERE for Sample Answers....TRY AND GIVE THE Q'S A GO FIRST!!
Labels:
Biology,
Microbiology,
Microorganism,
Revision,
Year 10 Sia1
Monday, September 6, 2010
Revision LInks for Year 10 A1: upcoming NCEA Exams
Guys...check the links below for some practice revision exams from the NCEA...these will be good to go over yourselves both for the upcoming Mock Exams and also the NCEA Exams in Term 4...
BIOLOGY
Describe Aspects of Biology 2007 Exam
Describe Aspects of Biology 2006 Exam
You can check your answers with these examplars links...aim for Excellence!
Excellence answers 2006
Merit answers 2006
Achievement answers 2006
CHEMISTRY
Describe Aspects of Chemistry 2007 Exam
Describe Aspects of Chemistry 2006 Exam
PHYSICS
Describe Aspects of Physics 2007 Exam
Describe Aspects of Physics 2006 Exam
There are also a few useful Revision Posts aand links on the blog if you search for them..
BIOLOGY
Describe Aspects of Biology 2007 Exam
Describe Aspects of Biology 2006 Exam
You can check your answers with these examplars links...aim for Excellence!
Excellence answers 2006
Merit answers 2006
Achievement answers 2006
CHEMISTRY
Describe Aspects of Chemistry 2007 Exam
Describe Aspects of Chemistry 2006 Exam
PHYSICS
Describe Aspects of Physics 2007 Exam
Describe Aspects of Physics 2006 Exam
There are also a few useful Revision Posts aand links on the blog if you search for them..
Tuesday, August 31, 2010
DC CIRCUITS REVISION
Check out this link and play around with it and have a go at contructing circuits...
DC CIRCUIT SIMULATION
There are plenty of other good educational (and FUN) simulations to play around with...
SCIENCE SIMULATIONS FROM THE UNIVERSITY OF COLORADO
DC CIRCUIT SIMULATION
There are plenty of other good educational (and FUN) simulations to play around with...
SCIENCE SIMULATIONS FROM THE UNIVERSITY OF COLORADO
Thursday, August 19, 2010
Forces and Mechanics for Year 10 Sia1 and 11 PY
At it's simplest a Force, symbol F can be seen as a push or a pull in a given direction. From this we can see that Force must be a Vector Quantity as it has a direction and also has a magnitude.
The Net Force, Fnet is the resultant sum of all the vector forces acting on a body and if these are balanced then Fnet is zero and the body is subject to Newton's First Law of Motion, ie it has inertia and remains in equilibrium:
- if it is at rest it will continue to remain at rest (be stationary) and if it is moving it will continue to move at a constant speed
- all the force vectors must add up to zero
If the forces become unbalanced the Fnet no longer add to zero and the the object starts to accelerate or decelerate, according to Newton's Second Law of Motion (Force = Mass x Acceleration) and the sum of all the vector forces must produce a resultant Force Vector in the direction of the acceleration.
Newton's third law can help us with understanding some of the other forces in play and how to represent them on a Force Diagram: "every action has an equal and opposite reaction." So for example if a box is sitting on the ground stationary there is a force, due to gravity, acting in a downward direction. From Newton's 3rd Law there must be an equal and opposite reaction: in this case a force acting upwards that is equal to the gravitational force: the two forces balance out with the resultant Fnett = 0, so the box remains stationary and will only move if another force acts on the box making the Fnett grater than zero. Now the forces are no longer in balance or equilibrium!
Labels:
Acceleration,
Forces,
Motion,
Newton's Laws of Motion,
Scalar,
Vectors,
Year 10 Sia1,
Year 11 PY
Monday, August 9, 2010
MOTION
A quick post to go over some of the main points of motion and the graphs associated with them. The first thing to get our heads around is the difference between scalar and vector quantities.
A Scalar quantity only has a magnitude whereas a Vector quantity has a direction as well as a magnitude.
For example time and distance are scalar quantities as they only have a magnitude, whereas displacement and velocity are vector quantities as they have both a magnitude and a direction:
Quantity Scalar or Vector Magnitude and Direction Unit
Displacement, as opposed to just distance, is the shortest distance form the start point and comprises a distance along with an angle or bearing, and Velocity is speed in a given direction.
 On the displacement time graph, to the side, the displacment is shown by the orange arrow, which shows the shortest distance from the origin. This displacement should be given with a distance in metres as well as a direction, with an angle or bearing.
On the displacement time graph, to the side, the displacment is shown by the orange arrow, which shows the shortest distance from the origin. This displacement should be given with a distance in metres as well as a direction, with an angle or bearing. On the velocity time graph the minus figure does not indicate a minus speed; instead it indicates a direction relative to the start direction which is shown from the origin. In this example if we assume the the y axis corresponds to a direction angle of 90 degrees or East, then the initial velocity is around 45 degrees, or North East, which then changes to approximately 135 degrees and returns to a direction / angle that is the same as the origin at 30 seconds. The velocity continues on this same angle / heading for another 7 seconds or so then changes angle back to a direction of approximately 45 degrees, or North East to return to the same direction / angle / bearing as the start direction / angle / bearing at 50 sec.
On the velocity time graph the minus figure does not indicate a minus speed; instead it indicates a direction relative to the start direction which is shown from the origin. In this example if we assume the the y axis corresponds to a direction angle of 90 degrees or East, then the initial velocity is around 45 degrees, or North East, which then changes to approximately 135 degrees and returns to a direction / angle that is the same as the origin at 30 seconds. The velocity continues on this same angle / heading for another 7 seconds or so then changes angle back to a direction of approximately 45 degrees, or North East to return to the same direction / angle / bearing as the start direction / angle / bearing at 50 sec.

A Scalar quantity only has a magnitude whereas a Vector quantity has a direction as well as a magnitude.
For example time and distance are scalar quantities as they only have a magnitude, whereas displacement and velocity are vector quantities as they have both a magnitude and a direction:
Quantity Scalar or Vector Magnitude and Direction Unit
Time Scalar Just a magnitude Seconds
Distance Scalar Just a magnitude Metres
Displacement Vector Magnitiude and Direction Metres
Velocity Vector Magnitude and Direction Metres per Second
Displacement, as opposed to just distance, is the shortest distance form the start point and comprises a distance along with an angle or bearing, and Velocity is speed in a given direction.
 On the displacement time graph, to the side, the displacment is shown by the orange arrow, which shows the shortest distance from the origin. This displacement should be given with a distance in metres as well as a direction, with an angle or bearing.
On the displacement time graph, to the side, the displacment is shown by the orange arrow, which shows the shortest distance from the origin. This displacement should be given with a distance in metres as well as a direction, with an angle or bearing. On the velocity time graph the minus figure does not indicate a minus speed; instead it indicates a direction relative to the start direction which is shown from the origin. In this example if we assume the the y axis corresponds to a direction angle of 90 degrees or East, then the initial velocity is around 45 degrees, or North East, which then changes to approximately 135 degrees and returns to a direction / angle that is the same as the origin at 30 seconds. The velocity continues on this same angle / heading for another 7 seconds or so then changes angle back to a direction of approximately 45 degrees, or North East to return to the same direction / angle / bearing as the start direction / angle / bearing at 50 sec.
On the velocity time graph the minus figure does not indicate a minus speed; instead it indicates a direction relative to the start direction which is shown from the origin. In this example if we assume the the y axis corresponds to a direction angle of 90 degrees or East, then the initial velocity is around 45 degrees, or North East, which then changes to approximately 135 degrees and returns to a direction / angle that is the same as the origin at 30 seconds. The velocity continues on this same angle / heading for another 7 seconds or so then changes angle back to a direction of approximately 45 degrees, or North East to return to the same direction / angle / bearing as the start direction / angle / bearing at 50 sec.Finally to reiterate a number of final points:
The gradient of a distance time graph equals the speed- The gradient of a velocity time graph equals the acceleration
- The area under a velocity time graph equals the distance travelled

Labels:
Acceleration,
Distance,
Gradient,
Graphs,
Motion,
Physics,
Scalar,
Time,
Vectors,
Year 10 Sia1,
Year 11 PY
Monday, July 26, 2010
FACTORS AFFECTING THE RATE OF CHEMICAL REACTION
From previous classes we know that: Reactants------------------> Products, or a number of reactants combine to form a product / products. We can also say that the particles in the products collide in order for a reactant to be formed.
Depending on the types of reactant the time taken for a reaction to occur can vary considerably.
For example, the chemical reactions that occur in the formation of crude oil occur over huge periods of time whereas the chemical reactions betwen Sodium and Acid occur very quickly. So how do we determine / measure the rate of a reaction? We can say:
Rate of Reaction = amount of reactant(s) used or product(s) formed / Time Taken
We can look at either how much reactant is used up in the chemical reaction or the amount of product formed. Below is a simplified graph to show this relationship:
It can be seen that with higher temperatures and higher concentration and smaller pieces the line of the graph showing the relationship between the total amount of products used and the time taken is steeper, thus indicating the reaction is happening more quickly.
Equally if this graph was showing the total amount of reactants being used against time then the steeper line would, again, indicate, a faster rate of reaction.
What this graph tells us then is that there are a number of factors that determine the rate of the reaction. These are listed below:
an excellent site with more on this as well as lots of other science topics
Depending on the types of reactant the time taken for a reaction to occur can vary considerably.
For example, the chemical reactions that occur in the formation of crude oil occur over huge periods of time whereas the chemical reactions betwen Sodium and Acid occur very quickly. So how do we determine / measure the rate of a reaction? We can say:
Rate of Reaction = amount of reactant(s) used or product(s) formed / Time Taken
We can look at either how much reactant is used up in the chemical reaction or the amount of product formed. Below is a simplified graph to show this relationship:
It can be seen that with higher temperatures and higher concentration and smaller pieces the line of the graph showing the relationship between the total amount of products used and the time taken is steeper, thus indicating the reaction is happening more quickly.
Equally if this graph was showing the total amount of reactants being used against time then the steeper line would, again, indicate, a faster rate of reaction.
What this graph tells us then is that there are a number of factors that determine the rate of the reaction. These are listed below:
- The temperature at which the reaction takes place
- The concentration of reactants being used
- The pressure at which the reaction is occuring
- The size of the pieces of reactants being used in the reaction
- The addition of a catalyst
- the higher the temperature the faster the rate of reaction
- the greater the concentration of dissolved reactants the faster the rate of reaction
- The higher the pressure of a reactsnt gas the faster the rate of reaction
- the smaller the pieces of a solid reactant the faster the rate of reaction
- if a catalyst is added the faster the rate of reaction
- with higher temperatures the greater the energy of the particles in the products and the more likely they are to react when they collide with other particles
- with greater concentrations of particles or pressure in gases the greater number of particles there are thereby increasing the chances of collisions at high enough energy levels to create a reaction
- with smaller pieces of solids both the surface area and the number of pieces are increased, thereby increasing the number of collisions which will speed up the chemical reaction
- if a catalyst is added the activation energy needed to result in a reaction when particles collide is lowered, thereby increasing the rate of the reaction. Catalysts are added to a reaction but are not used up
an excellent site with more on this as well as lots of other science topics
Thursday, July 8, 2010
GRAPHING TUTORIAL
 When having to draw graphs and interpret data the starting point is making sure the correct axes are used. The independent variable goes on the X axis (horizontal axis) and the dependent variable goes on the y axis (vertical axis).
When having to draw graphs and interpret data the starting point is making sure the correct axes are used. The independent variable goes on the X axis (horizontal axis) and the dependent variable goes on the y axis (vertical axis).From designing our Fair Tests we know that the Independent Variable is the variable that we, as Scientists, change, making sure that we only use the 1 independent variable. As we change the value of the independent variable we observe what happens to the Dependent Variable. If we have designed a good Fair Test then there should be a relationship between the two; this is what we are testing. Time is often an Independent variable that is used with observations being observed for any changes in the chosen dependent variable over time.
For example we could look at changes in rate of growth of microorganisms on an agar plate over time. With all other variables being controlled, such as temperature, nutrient source, sterilisation etc then time which is obviously measurable is the independent variable and changes in growth is the dependent variable. If we changed the fair test to study the effects of different concentrations of disinfectant on microorganism growth then the concentrations of disinfectant we have chosen, and measured, would be the independent variable.
Returning to graphs there are a number of types of graphs we can draw depending upon the type of data being analysed. Some of the most common include:
- Line Graphs
- Bar Charts
- Pie Charts
The science buddies website has more useful information:
this page gives a good overview and lists key points when drawing up graphs!
Monday, June 28, 2010
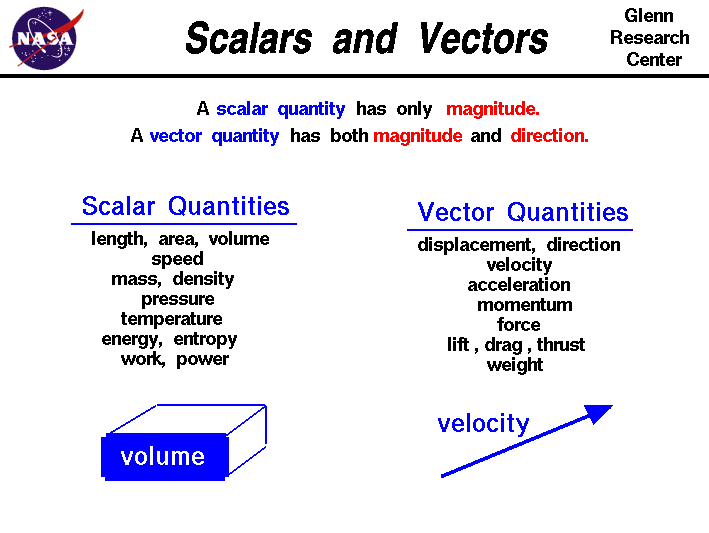
SCALAR AND VECTOR QUANTITIES
As can be seen from the diagram Scalar quantities only have a magnitude whereas Vector quantities have a direction and a magnitude. Therefore speed for example is a scalar magnitude whereas velocity is a vector quantity because it has both a magnitude and a direction; velocity is speed in a given direction
CLICK HERE FOR MORE INFO ON VECTORS
CLICK HERE FOR MORE INFO ON VECTORS
Tuesday, June 22, 2010
METAL REACTIVITY SERIES AND DISPLACEMENT REACTIONS 2
As a follow on form the previous post here is a bit more info...
DISPLACEMENT REACTION IN SOLUTION
A more reactive metal will displace a metal from it's compound in solution.
Observations could include:
DISPLACEMENT REACTION IN SOLUTION
A more reactive metal will displace a metal from it's compound in solution.
Observations could include:
- the more reactive metal gradually dissolves
- the less reactive metal coats the more reactive metal
- the solution may change colour
- theat is given out because these reactions are exothermic
- fizzing may occur
- Magnesium and Copper Sulphate solution
The Word equation is:
Magnesium + Copper Sulphate
---------> Magnesium Sulphate + Copper
The Magnesium has been coated with Copper in this displacement reaction
2. Iron and Copper Sulphate Solution
The word equation for this reaction is:
Iron + Copper Sulphate
------------> Iron Sulphate + Copper
The Iron becomes coated with Copper in this displacement reaction
However the rate of this reaction is much slower (it takes much longer) as Iron and Copper are much closer together in the Reactivity Series
Metal Compound in Solution
MgSo4 CuSo4 FeSo4
Mg No Yes Yes
Cu No No No
Fe No Yes No
A metal will not react with it's compound
Magnesium is the most reactive of the three because it reacted with the other two metals
Copper is the least reactive because it didn't react with compounds of the other two metals
Note that the compound itself is not important, only the metal it contains, so Copper Nitrate, for example, would give the same answer as Copper Sulphate
Displacement reactions also occur with solid metal oxides, but that is for another post.
.
Labels:
Chemistry,
Displacement Reactions,
Metals,
Reactivity Series,
Year 11 PY
Monday, June 21, 2010
METAL REACTIVITY AND DISPLACEMENT REACTIONS
 This post should help with Year 11PY internal assessment 1.1
This post should help with Year 11PY internal assessment 1.1The reactivity series to the side shows a list of metals, with the addition of two non metals: carbon and hydrogen, arranged in order of their reactivity from most reactive to least reactive.By using the reactivity series we can predict what will happen in displacement reactions. The reason carbon and hydrogen are included in this list is because carbon is used in the extraction of iron ore and any metals below hydrogen in the list will not react with dilute HCl.
Metals react by losing electrons which is oxidation; metals are the reductant, ie they donate electrons.
When metals react with other other metals in a metal salt solution a displacement reaction takes place; a more reactive metal will take the place of a less reactive metal. In other words the more reactive metal donates electrons to the less reactive metal (existing in ion form).
The key point is that if a less reactive metal is added to a metal salt solution which contains metal ions from a more reactive metal then there will be no displacement and no reaction will take place. Therefore if we put Copper, Lead, Zinc, Aluminium into a solution of Magnesium Sulphate as Magnesium is more reactive there will be no reaction and nothing to observe as the Magnesium 'wins the competition' for the Sulphate ions.
Other Metal Salts that can be used are Nitrates and Chlorides for example.
In the practical carried out another example may help in consolidating knowledge. If we take Zinc and a solution containing Copper ions, in this case Copper Sulphate what is going to happen?
- Looking at the reactivity series above it can be seen that Zinc is more reactive than Copper
- Zinc being more reactive forces the Copper ions to accept electrons, ie Zinc acts as a reductant by donating electrons to the Copper ions in solution, which become Copper metal atoms
- Zinc then accepts the Sulphate ions and becomes oxidised and becomes Zinc Sulphate
- The Zinc metal will discolour and the blue Copper Sulphate solution fades in colour
- We can say that Zinc has displaced the Copper ions from the Copper Sulphate solution
- Zn -----------------> Zn2+ + 2e
- C2+ + 2e ----------------> Cu
Using this information and the reactivity series you should be able to work out what metals will displace other metal ions from a metal salt solution
Labels:
Displacement Reactions,
Ions,
Metals,
Oxidation,
Reactivity Series,
Reduction,
Year 11 PY
Sunday, June 20, 2010
DEVISING A FAIR TEST WHEN DOING A PRACTICAL
This revision post should interest both Year 10 SiA1 and 11 PY as both classes are involved in doing practical investigations with direction; ie designing and implementing and writing up an experiment. Before carrying out the actual practical assessment the opportunity is being given for a practice run.
This link gives a simplified step by step guide to designing a fair test
The main points are:
- in the case of year 10 the practice is to do with designing a Fair Test to investigate something to do with the swing of a pendulum. This will be followed up by another practice, this time called Hot Wheels
- in the case of year 11 the practice will be with a Metal Activity Series
This link gives a simplified step by step guide to designing a fair test
The main points are:
- Come up with an Hypothesis / Statement against which you are going to implement your Fair Test. If it is a statement this should be a statement of what you think is going to happen
- Identify the following variables: independent, dependent and control variables and make sure to list them all stating which type of variable they are
- The independent variable, which is plotted on the x axis, is the variable you have identified in your hypothesis as affecting the dependent variable, and is the ONE variable that you are going to change
- The dependent variable is the variable that is going to change as a result of you changing the independent variable, and is plotted on the y axis
- The control variables are all the other variables that could affect the fair test if you do not 'control' them, ensuring they are always the same
- Make sure that a full step step methodology is written down in your Fair Test stating how you are going to ensure that the test is fair, ie how you are going to measure the independent variable making sure that all the other control variables are taken into account and kept the same throughout the test. This methodology should be easy and simple enough for another group of students to pick up and carry out the same experiment you have just written and carried out!
- Make a table of observed results and a graph if appropriate
- Draw up your conclusions and evaluate whether the Fair Test you carried out was fair and either proved or disproved your original statement/hypothesis. Bear in mind that depending on your original statement you may or may not agree with the hypothesis; this is alright as long as you have carried out a fair test. If things did not work out as expected then your evaluation should include what you would change, or do differently, next time
Saturday, June 19, 2010
CHEMICAL REACTIONS: OXIDATION AND REDUCTION...10Sia1
REDOX reactions or Oxidation and Reduction Reactions occur in two parts: Reduction and Oxidation
Reduction
In general this involves the gain of electrons or loss of oxygen. The atom, molecule or compound that gains electrons is known as the Oxidant
Oxidation
In general this involves the loss of electrons or gain of oxygen. The atom, molecule or compound that loses electrons or gains oxygen is known as the reductant
Oxidation and Reduction always occur together and there is no net change in the charge. The electrons lost by the reductant must equal the electrons gained by the oxidant, ie they must balance!
Here is a worked example:
H2 + F2 ---------------> 2HF
Reduction
In general this involves the gain of electrons or loss of oxygen. The atom, molecule or compound that gains electrons is known as the Oxidant
Oxidation
In general this involves the loss of electrons or gain of oxygen. The atom, molecule or compound that loses electrons or gains oxygen is known as the reductant
Oxidation and Reduction always occur together and there is no net change in the charge. The electrons lost by the reductant must equal the electrons gained by the oxidant, ie they must balance!
Here is a worked example:
H2 + F2 ---------------> 2HF
- the first step is to separate the equation into 2 half reactions:
- H2 → 2 H+ + 2 e− (the oxidation reaction)
- F2 + 2 e− → 2 F− (the reduction reaction)
- When the 2 half equations are combined there is no net change in charges and the Hydrogen and Flourine Ions combine to form Hydrogen Fluorine
- H2 + F2 → 2 H+ + 2 F− → 2 HF
A flash tutorial on oxidation and reduction
Friday, June 18, 2010
Global Warming relevant to 12 Si June 2010
What is a global pattern? At its simplest it could be described as anything that happens around the globe in a similar manner. The diagram to the side shows wind movements between 30 degrees South and North; these can be described as global in nature as they occur on a global scale.
Other examples of global patterns could include: global patterns of earthquake distributions(seismic activity) and volcanic eruptions. Again, similar occurrences occur around the globe from New Zealand to Iceland and in between, all related to the pattern of the earth's crust and the tectonic plates, for example the Pacific Ring of Fire.
Returning to the wind patterns these are part of the global climate patterns which arise from the differential heating of the earth's oceans and land masses by the sun and the transfer of this heat energy through global circulations through the atmosphere and the oceans, and the links between the two. The land temperatures fluctuates much more quickly and over a wider temperature range compared to the oceans which act like a heat sink.
As mentioned the earth receives its heat from the sun in the form of UV and visible light photons which heat the land and oceans. The land irradiates some of this heat back to space. If all the heat radiated from the sun to the earth was irradiated back to space then the planet would be too cold to support life. However the gases in the atmosphere trap some of this Infra red radiation, thus warming up the temperature of the earth, allowing life to survive and flourish; this is known as the greenhouse effect which is again another global pattern. Without the greenhouse effect there would be no life on earth; you can compare it to a greenhouse where the glass allows sunlight energy in but traps a lot of the heat inside the greenhouse allowing a horticulturist to grow fruits and vegetables that s/he would not be able to grow outside. Another analogy would be to think of sitting in a car with the windows up on a hot summer day and slowly baking as the heat becomes oppressive! Not a nice place!
This is where the global pattern: global warming enters the picture; and not in a good way for many of aspects of the earth's human and non human facets, which are all interconnected, or linked, in some way or another.At it's simplest if can be understood that the natural occurring greenhouse effect is having an adverse effect on global climate patterns by causing the average temperature of the earth to increase, due in part to human activity and the ever increasing technological race and exploitation of the planet as humans have gone from a rural agrarian lifestyle to a more urban, complex and technology advanced lifestyle. Factors to consider are increasing exploitation and use of fossil fuels for transport, heat, electricity production, industrialisation etc which has gone hand in hand with ever increasing rates of deforestation.
It may be easy to think of global patterns in terms of territories, borders and flows, and proximity and distance in order to understand some of the links and processes within this pattern. It is obvious that New Zealand and any other country can be classified as a territory but so can fossil fuel reserves, polar ice caps, wind patterns, oceans, areas of forest such as the Amazon, carbon dioxide emissions, the atmosphere etc, whilst flows can include movements of people from territory to territory or just within a territory, as well as, for example, flows of carbon dioxide from one territory to another. As things can flow they can then obviously have impacts across wide areas; in this case globally because increasingly human flows are becoming less and less constrained by borders, non human flows are not subject to border controls and some territories, such as oceans and the atmosphere, are not constrained by borders.
I hope this post will help in preparing for the Unit Standard Assessment (5093); it should be a start anyway.
Other examples of global patterns could include: global patterns of earthquake distributions(seismic activity) and volcanic eruptions. Again, similar occurrences occur around the globe from New Zealand to Iceland and in between, all related to the pattern of the earth's crust and the tectonic plates, for example the Pacific Ring of Fire.
Returning to the wind patterns these are part of the global climate patterns which arise from the differential heating of the earth's oceans and land masses by the sun and the transfer of this heat energy through global circulations through the atmosphere and the oceans, and the links between the two. The land temperatures fluctuates much more quickly and over a wider temperature range compared to the oceans which act like a heat sink.
As mentioned the earth receives its heat from the sun in the form of UV and visible light photons which heat the land and oceans. The land irradiates some of this heat back to space. If all the heat radiated from the sun to the earth was irradiated back to space then the planet would be too cold to support life. However the gases in the atmosphere trap some of this Infra red radiation, thus warming up the temperature of the earth, allowing life to survive and flourish; this is known as the greenhouse effect which is again another global pattern. Without the greenhouse effect there would be no life on earth; you can compare it to a greenhouse where the glass allows sunlight energy in but traps a lot of the heat inside the greenhouse allowing a horticulturist to grow fruits and vegetables that s/he would not be able to grow outside. Another analogy would be to think of sitting in a car with the windows up on a hot summer day and slowly baking as the heat becomes oppressive! Not a nice place!
This is where the global pattern: global warming enters the picture; and not in a good way for many of aspects of the earth's human and non human facets, which are all interconnected, or linked, in some way or another.At it's simplest if can be understood that the natural occurring greenhouse effect is having an adverse effect on global climate patterns by causing the average temperature of the earth to increase, due in part to human activity and the ever increasing technological race and exploitation of the planet as humans have gone from a rural agrarian lifestyle to a more urban, complex and technology advanced lifestyle. Factors to consider are increasing exploitation and use of fossil fuels for transport, heat, electricity production, industrialisation etc which has gone hand in hand with ever increasing rates of deforestation.
It may be easy to think of global patterns in terms of territories, borders and flows, and proximity and distance in order to understand some of the links and processes within this pattern. It is obvious that New Zealand and any other country can be classified as a territory but so can fossil fuel reserves, polar ice caps, wind patterns, oceans, areas of forest such as the Amazon, carbon dioxide emissions, the atmosphere etc, whilst flows can include movements of people from territory to territory or just within a territory, as well as, for example, flows of carbon dioxide from one territory to another. As things can flow they can then obviously have impacts across wide areas; in this case globally because increasingly human flows are becoming less and less constrained by borders, non human flows are not subject to border controls and some territories, such as oceans and the atmosphere, are not constrained by borders.
I hope this post will help in preparing for the Unit Standard Assessment (5093); it should be a start anyway.
Subscribe to:
Posts (Atom)