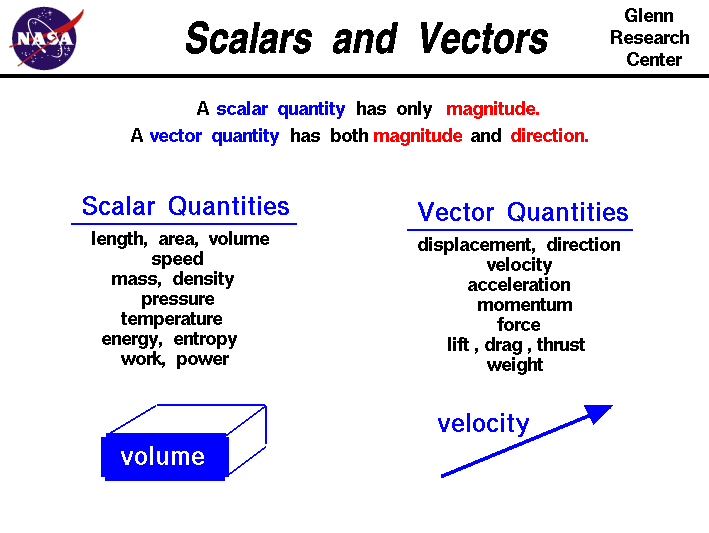
As can be seen from the diagram Scalar quantities only have a magnitude whereas Vector quantities have a direction and a magnitude. Therefore speed for example is a scalar magnitude whereas velocity is a vector quantity because it has both a magnitude and a direction; velocity is speed in a given direction
CLICK HERE FOR MORE INFO ON VECTORS
School Banner

School Logo
Monday, June 28, 2010
Tuesday, June 22, 2010
METAL REACTIVITY SERIES AND DISPLACEMENT REACTIONS 2
As a follow on form the previous post here is a bit more info...
DISPLACEMENT REACTION IN SOLUTION
A more reactive metal will displace a metal from it's compound in solution.
Observations could include:
DISPLACEMENT REACTION IN SOLUTION
A more reactive metal will displace a metal from it's compound in solution.
Observations could include:
- the more reactive metal gradually dissolves
- the less reactive metal coats the more reactive metal
- the solution may change colour
- theat is given out because these reactions are exothermic
- fizzing may occur
- Magnesium and Copper Sulphate solution
The Word equation is:
Magnesium + Copper Sulphate
---------> Magnesium Sulphate + Copper
The Magnesium has been coated with Copper in this displacement reaction
2. Iron and Copper Sulphate Solution
The word equation for this reaction is:
Iron + Copper Sulphate
------------> Iron Sulphate + Copper
The Iron becomes coated with Copper in this displacement reaction
However the rate of this reaction is much slower (it takes much longer) as Iron and Copper are much closer together in the Reactivity Series
Metal Compound in Solution
MgSo4 CuSo4 FeSo4
Mg No Yes Yes
Cu No No No
Fe No Yes No
A metal will not react with it's compound
Magnesium is the most reactive of the three because it reacted with the other two metals
Copper is the least reactive because it didn't react with compounds of the other two metals
Note that the compound itself is not important, only the metal it contains, so Copper Nitrate, for example, would give the same answer as Copper Sulphate
Displacement reactions also occur with solid metal oxides, but that is for another post.
.
Labels:
Chemistry,
Displacement Reactions,
Metals,
Reactivity Series,
Year 11 PY
Monday, June 21, 2010
METAL REACTIVITY AND DISPLACEMENT REACTIONS
 This post should help with Year 11PY internal assessment 1.1
This post should help with Year 11PY internal assessment 1.1The reactivity series to the side shows a list of metals, with the addition of two non metals: carbon and hydrogen, arranged in order of their reactivity from most reactive to least reactive.By using the reactivity series we can predict what will happen in displacement reactions. The reason carbon and hydrogen are included in this list is because carbon is used in the extraction of iron ore and any metals below hydrogen in the list will not react with dilute HCl.
Metals react by losing electrons which is oxidation; metals are the reductant, ie they donate electrons.
When metals react with other other metals in a metal salt solution a displacement reaction takes place; a more reactive metal will take the place of a less reactive metal. In other words the more reactive metal donates electrons to the less reactive metal (existing in ion form).
The key point is that if a less reactive metal is added to a metal salt solution which contains metal ions from a more reactive metal then there will be no displacement and no reaction will take place. Therefore if we put Copper, Lead, Zinc, Aluminium into a solution of Magnesium Sulphate as Magnesium is more reactive there will be no reaction and nothing to observe as the Magnesium 'wins the competition' for the Sulphate ions.
Other Metal Salts that can be used are Nitrates and Chlorides for example.
In the practical carried out another example may help in consolidating knowledge. If we take Zinc and a solution containing Copper ions, in this case Copper Sulphate what is going to happen?
- Looking at the reactivity series above it can be seen that Zinc is more reactive than Copper
- Zinc being more reactive forces the Copper ions to accept electrons, ie Zinc acts as a reductant by donating electrons to the Copper ions in solution, which become Copper metal atoms
- Zinc then accepts the Sulphate ions and becomes oxidised and becomes Zinc Sulphate
- The Zinc metal will discolour and the blue Copper Sulphate solution fades in colour
- We can say that Zinc has displaced the Copper ions from the Copper Sulphate solution
- Zn -----------------> Zn2+ + 2e
- C2+ + 2e ----------------> Cu
Using this information and the reactivity series you should be able to work out what metals will displace other metal ions from a metal salt solution
Labels:
Displacement Reactions,
Ions,
Metals,
Oxidation,
Reactivity Series,
Reduction,
Year 11 PY
Sunday, June 20, 2010
DEVISING A FAIR TEST WHEN DOING A PRACTICAL
This revision post should interest both Year 10 SiA1 and 11 PY as both classes are involved in doing practical investigations with direction; ie designing and implementing and writing up an experiment. Before carrying out the actual practical assessment the opportunity is being given for a practice run.
This link gives a simplified step by step guide to designing a fair test
The main points are:
- in the case of year 10 the practice is to do with designing a Fair Test to investigate something to do with the swing of a pendulum. This will be followed up by another practice, this time called Hot Wheels
- in the case of year 11 the practice will be with a Metal Activity Series
This link gives a simplified step by step guide to designing a fair test
The main points are:
- Come up with an Hypothesis / Statement against which you are going to implement your Fair Test. If it is a statement this should be a statement of what you think is going to happen
- Identify the following variables: independent, dependent and control variables and make sure to list them all stating which type of variable they are
- The independent variable, which is plotted on the x axis, is the variable you have identified in your hypothesis as affecting the dependent variable, and is the ONE variable that you are going to change
- The dependent variable is the variable that is going to change as a result of you changing the independent variable, and is plotted on the y axis
- The control variables are all the other variables that could affect the fair test if you do not 'control' them, ensuring they are always the same
- Make sure that a full step step methodology is written down in your Fair Test stating how you are going to ensure that the test is fair, ie how you are going to measure the independent variable making sure that all the other control variables are taken into account and kept the same throughout the test. This methodology should be easy and simple enough for another group of students to pick up and carry out the same experiment you have just written and carried out!
- Make a table of observed results and a graph if appropriate
- Draw up your conclusions and evaluate whether the Fair Test you carried out was fair and either proved or disproved your original statement/hypothesis. Bear in mind that depending on your original statement you may or may not agree with the hypothesis; this is alright as long as you have carried out a fair test. If things did not work out as expected then your evaluation should include what you would change, or do differently, next time
Saturday, June 19, 2010
CHEMICAL REACTIONS: OXIDATION AND REDUCTION...10Sia1
REDOX reactions or Oxidation and Reduction Reactions occur in two parts: Reduction and Oxidation
Reduction
In general this involves the gain of electrons or loss of oxygen. The atom, molecule or compound that gains electrons is known as the Oxidant
Oxidation
In general this involves the loss of electrons or gain of oxygen. The atom, molecule or compound that loses electrons or gains oxygen is known as the reductant
Oxidation and Reduction always occur together and there is no net change in the charge. The electrons lost by the reductant must equal the electrons gained by the oxidant, ie they must balance!
Here is a worked example:
H2 + F2 ---------------> 2HF
Reduction
In general this involves the gain of electrons or loss of oxygen. The atom, molecule or compound that gains electrons is known as the Oxidant
Oxidation
In general this involves the loss of electrons or gain of oxygen. The atom, molecule or compound that loses electrons or gains oxygen is known as the reductant
Oxidation and Reduction always occur together and there is no net change in the charge. The electrons lost by the reductant must equal the electrons gained by the oxidant, ie they must balance!
Here is a worked example:
H2 + F2 ---------------> 2HF
- the first step is to separate the equation into 2 half reactions:
- H2 → 2 H+ + 2 e− (the oxidation reaction)
- F2 + 2 e− → 2 F− (the reduction reaction)
- When the 2 half equations are combined there is no net change in charges and the Hydrogen and Flourine Ions combine to form Hydrogen Fluorine
- H2 + F2 → 2 H+ + 2 F− → 2 HF
A flash tutorial on oxidation and reduction
Friday, June 18, 2010
Global Warming relevant to 12 Si June 2010
What is a global pattern? At its simplest it could be described as anything that happens around the globe in a similar manner. The diagram to the side shows wind movements between 30 degrees South and North; these can be described as global in nature as they occur on a global scale.
Other examples of global patterns could include: global patterns of earthquake distributions(seismic activity) and volcanic eruptions. Again, similar occurrences occur around the globe from New Zealand to Iceland and in between, all related to the pattern of the earth's crust and the tectonic plates, for example the Pacific Ring of Fire.
Returning to the wind patterns these are part of the global climate patterns which arise from the differential heating of the earth's oceans and land masses by the sun and the transfer of this heat energy through global circulations through the atmosphere and the oceans, and the links between the two. The land temperatures fluctuates much more quickly and over a wider temperature range compared to the oceans which act like a heat sink.
As mentioned the earth receives its heat from the sun in the form of UV and visible light photons which heat the land and oceans. The land irradiates some of this heat back to space. If all the heat radiated from the sun to the earth was irradiated back to space then the planet would be too cold to support life. However the gases in the atmosphere trap some of this Infra red radiation, thus warming up the temperature of the earth, allowing life to survive and flourish; this is known as the greenhouse effect which is again another global pattern. Without the greenhouse effect there would be no life on earth; you can compare it to a greenhouse where the glass allows sunlight energy in but traps a lot of the heat inside the greenhouse allowing a horticulturist to grow fruits and vegetables that s/he would not be able to grow outside. Another analogy would be to think of sitting in a car with the windows up on a hot summer day and slowly baking as the heat becomes oppressive! Not a nice place!
This is where the global pattern: global warming enters the picture; and not in a good way for many of aspects of the earth's human and non human facets, which are all interconnected, or linked, in some way or another.At it's simplest if can be understood that the natural occurring greenhouse effect is having an adverse effect on global climate patterns by causing the average temperature of the earth to increase, due in part to human activity and the ever increasing technological race and exploitation of the planet as humans have gone from a rural agrarian lifestyle to a more urban, complex and technology advanced lifestyle. Factors to consider are increasing exploitation and use of fossil fuels for transport, heat, electricity production, industrialisation etc which has gone hand in hand with ever increasing rates of deforestation.
It may be easy to think of global patterns in terms of territories, borders and flows, and proximity and distance in order to understand some of the links and processes within this pattern. It is obvious that New Zealand and any other country can be classified as a territory but so can fossil fuel reserves, polar ice caps, wind patterns, oceans, areas of forest such as the Amazon, carbon dioxide emissions, the atmosphere etc, whilst flows can include movements of people from territory to territory or just within a territory, as well as, for example, flows of carbon dioxide from one territory to another. As things can flow they can then obviously have impacts across wide areas; in this case globally because increasingly human flows are becoming less and less constrained by borders, non human flows are not subject to border controls and some territories, such as oceans and the atmosphere, are not constrained by borders.
I hope this post will help in preparing for the Unit Standard Assessment (5093); it should be a start anyway.
Other examples of global patterns could include: global patterns of earthquake distributions(seismic activity) and volcanic eruptions. Again, similar occurrences occur around the globe from New Zealand to Iceland and in between, all related to the pattern of the earth's crust and the tectonic plates, for example the Pacific Ring of Fire.
Returning to the wind patterns these are part of the global climate patterns which arise from the differential heating of the earth's oceans and land masses by the sun and the transfer of this heat energy through global circulations through the atmosphere and the oceans, and the links between the two. The land temperatures fluctuates much more quickly and over a wider temperature range compared to the oceans which act like a heat sink.
As mentioned the earth receives its heat from the sun in the form of UV and visible light photons which heat the land and oceans. The land irradiates some of this heat back to space. If all the heat radiated from the sun to the earth was irradiated back to space then the planet would be too cold to support life. However the gases in the atmosphere trap some of this Infra red radiation, thus warming up the temperature of the earth, allowing life to survive and flourish; this is known as the greenhouse effect which is again another global pattern. Without the greenhouse effect there would be no life on earth; you can compare it to a greenhouse where the glass allows sunlight energy in but traps a lot of the heat inside the greenhouse allowing a horticulturist to grow fruits and vegetables that s/he would not be able to grow outside. Another analogy would be to think of sitting in a car with the windows up on a hot summer day and slowly baking as the heat becomes oppressive! Not a nice place!
This is where the global pattern: global warming enters the picture; and not in a good way for many of aspects of the earth's human and non human facets, which are all interconnected, or linked, in some way or another.At it's simplest if can be understood that the natural occurring greenhouse effect is having an adverse effect on global climate patterns by causing the average temperature of the earth to increase, due in part to human activity and the ever increasing technological race and exploitation of the planet as humans have gone from a rural agrarian lifestyle to a more urban, complex and technology advanced lifestyle. Factors to consider are increasing exploitation and use of fossil fuels for transport, heat, electricity production, industrialisation etc which has gone hand in hand with ever increasing rates of deforestation.
It may be easy to think of global patterns in terms of territories, borders and flows, and proximity and distance in order to understand some of the links and processes within this pattern. It is obvious that New Zealand and any other country can be classified as a territory but so can fossil fuel reserves, polar ice caps, wind patterns, oceans, areas of forest such as the Amazon, carbon dioxide emissions, the atmosphere etc, whilst flows can include movements of people from territory to territory or just within a territory, as well as, for example, flows of carbon dioxide from one territory to another. As things can flow they can then obviously have impacts across wide areas; in this case globally because increasingly human flows are becoming less and less constrained by borders, non human flows are not subject to border controls and some territories, such as oceans and the atmosphere, are not constrained by borders.
I hope this post will help in preparing for the Unit Standard Assessment (5093); it should be a start anyway.
Subscribe to:
Posts (Atom)